If you have ever wondered why GLP-1s are dominating every conversation from doctors’ rooms to dinner tables, this article by Dr Heidi Frere explains it beautifully. She unpacks the science, the safety, the South African landscape and where this entire field is heading without any hype. Consider this your clear and medically grounded guide to understanding one of the most important developments in weight management and longevity medicine today.

Are GLP-1 Agonists the Answer to the Global Metabolic Epidemic? First, let us discuss what they are:
Enter the fascinating world of peptides. GLP-1 agonists are not “weight-loss shots” or “quick fixes”.
They are a class of medications that mimic a natural gut hormone: Glucagon-Like Peptide-1, helping to regulate appetite, blood sugar and weight.
These medications have transformed both diabetes and weight-management care, ushering in a new era of metabolic and longevity medicine. They help to regulate appetite, blood sugar and weight.
And, yes, they are here to stay!
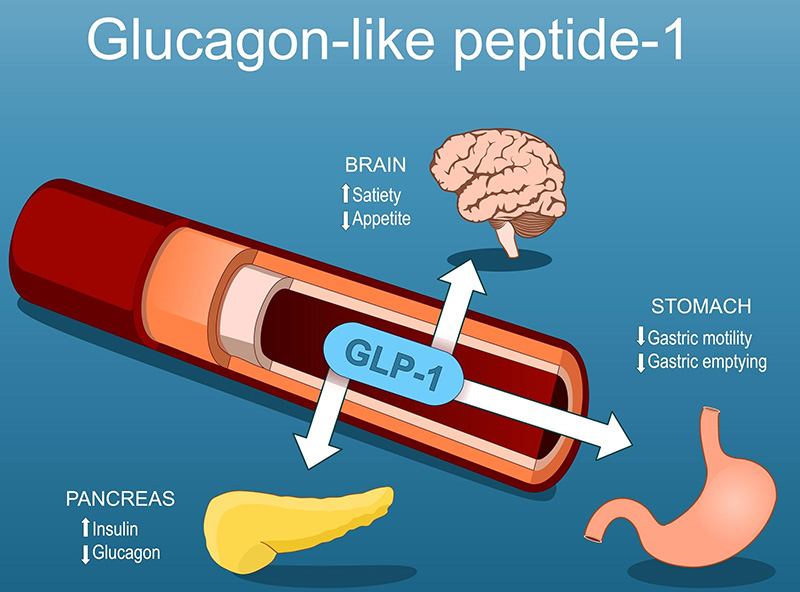
How GLP-1s Work
The GLP-1 agonists mimic a naturally occurring hormone. Again, science has taken a feather from Mother Nature’s cap. It is called an incretin hormone.
Incretins are produced by our intestinal cells in response to food. Hormones and peptides act like messengers. These messages are master communicators sent to the pancreas, brain, stomach and liver, all with the purpose of using energy more efficiently. They have a profound impact by modulating insulin and glucose metabolism. Beyond their role in diabetes and weight management, they are likely to have potential in anti-ageing and longevity medicine.
They allow the pancreas to release insulin only when glucose levels are elevated. They suppress the production of glucagon, which raises blood sugar. The outcome is better glucose-level control after meals.
At the level of the brain, they reduce appetite and food cravings. Patients report a reduction in “food noise” and early satiety after or during a meal. Interestingly, in my practice, patients have also reported a reduction in cravings for alcohol and cigarettes. Though anecdotal, an overall improvement in addictive behaviours has been reported. The overall result is less caloric intake and weight loss.
At the level of the stomach, there is delayed gastric emptying and food moves more slowly from the stomach into the intestine, which helps with a prolonged feeling of fullness. A potential concern was that of gastroparesis (the muscles of the stomach will no longer work well), but this concern has largely been eliminated by the research evidence, while appetite suppression with long-term use remains.
At the level of the liver and fat tissue, less glucose is put out into the system. Insulin sensitivity is improved, and there is a reduction in visceral fat and non-alcoholic fatty liver disease (NAFLD). This has a profound effect on improving metabolic health and reducing cardiovascular disease.
In essence, GLP-1 agonists act on multiple organs: the brain, pancreas, stomach and liver to improve metabolic efficiency, reduce hunger and protect against chronic disease.
| Target System | Primary Effect | Clinical Result |
| Pancreas | ↑ Insulin, ↓ Glucagon | Improved glucose control |
| Brain | Appetite suppression | Weight loss |
| Stomach | Slower emptying | Early satiety |
| Liver | ↓ Glucose output | Reduced insulin resistance |
| Heart & Vessels | Anti-inflammatory, anti-atherogenic | Cardiovascular benefit |
Research-backed benefits
Cardiovascular and renal protection by lowering blood pressure, reducing inflammation, improving endothelial cell function and lowering cholesterol.
As a result, the incidence of atherosclerotic disease in patients on GLP-1 agonists will decline. Studies like LEADER, SUSTAIN-6, REWIND, MACE: together, these studies consistently demonstrated reduced cardiovascular risk, improved glycaemic control and, in some cases, lower all-cause mortality.
| Trial (Year) | Drug | Population | Primary CV result (3-pt MACE) |
| LEADER (2016) | Liraglutide (daily) | T2D at high CV risk | HR 0.87 vs placebo (significant CV benefit). (PMC) |
| SUSTAIN-6 (2016) | Semaglutide (weekly) | T2D, high risk | HR 0.74 vs placebo (significant). (Europe PMC) |
| PIONEER-6 (2019) | Semaglutide (oral) | T2D, high risk | Non-inferior to placebo for MACE (safety established). (New England Journal of Medicine) |
| REWIND (2019) | Dulaglutide (weekly) | T2D with/without prior CVD (broad risk) | HR 0.88 (significant) over median 5.4 yrs. (PubMed) |
| HARMONY Outcomes (2018) | Albiglutide (weekly) | T2D with established ASCVD | HR 0.78 (significant). (American College of Cardiology) |
| EXSCEL (2017) | Exenatide ER (weekly) | T2D with/without CVD | HR 0.91 (trend only; not statistically significant). (New England Journal of Medicine) |
| ELIXA (2015) | Lixisenatide (daily) | T2D post-ACS | HR 1.02 (non-inferior; no benefit). (New England Journal of Medicine) |
| AMPLITUDE-O(2021) | Efpeglenatide (weekly) | T2D with CVD or CKD | HR 0.73 (significant) for MACE; renal benefit also reported. (New England Journal of Medicine) |
| SELECT (2023) | Semaglutide 2.4 mg (weekly; obesity dose) | Overweight/obesity without diabetes, established CVD | HR 0.80 (significant) — first GLP-1 CV benefit in non-diabetic obesity. (PubMed) |
Brain-protection and cognitive benefits
There is a reduction in neuroinflammation and a process called tau hyperphosphorylation, which is a hallmark and very relevant to Alzheimer’s disease. Evidence suggests a delayed progression of cognitive decline and dementia in patients on GLP-1 agonists.
The psychological and mental health benefits for the obese and overweight population go without saying. Behavioural control and psychological relief were key reported benefits. However, they are not a stand-alone treatment for patients with mood disorders in the overweight patient.
Exploring the South African landscape
For anyone who thinks that GLP-1s have just arrived or are a new “fad” for quick weight loss, think again!
Here is a summary of all the GLP-1 agonists registered by SAHPRA (South African Health Products Regulatory Authority) and launched in South Africa. All this information is available in the product package inserts or online at www.sahpra.org.za
- Byetta® (Exenatide) was authorised in SA in 2008 and was available in 2 dosages. Locally held by AstraZeneca, this injectable product was injected twice daily and was very affordable. It was FDA (US-based Food and Drug Administration regulatory body) approved for Type 2 Diabetes. For doctors, quick to adopt medications for their weight-loss benefits, they used it off-label and it worked reasonably well, with the appropriate lifestyle change, albeit the inconvenience of injecting a refrigerated product twice daily. It was discontinued in February 2024.
- Bydureon BCise® (Exenatide ER), held by AstraZeneca, indicated for Type 2 Diabetes, was a once-weekly injection and never released in South Africa.
- Then came Victoza® (Liraglutide) made by Novo Nordisk, registered in SA in November 2011, also FDA approved for management of Type 2 Diabetes, an adjunct to diet and exercise. With a dose-adjusted pen with a 24-hour half-life and needing refrigeration. Used as monotherapy or in combination with other glucose-lowering medications with an emphasis on preventing cardiovascular disease.
- Then Trulicity® (Dulaglutide), Eli-Lilly, authorised in March 2020, was FDA approved for the management of Type 2 Diabetes, an adjunct to diet and exercise. A single-dose pen injected weekly was very convenient for those travelling. It does not require refrigeration. Used as monotherapy or in combination with other glucose-lowering medications with an emphasis on preventing cardiovascular disease.
- Then Saxenda® (Liraglutide) Novo Nordisk, with FDA approval for medically supervised weight-loss management, was registered in SA in March 2020. Indicated for patients with a BMI ≥ 30kg/m² (obese) or ≥ 27kg/m² (overweight) in the presence of at least 1 weight-associated co-morbidity such as sleep apnoea, hypertension, high cholesterol or dyslipidaemia, dysglycaemia or metabolic syndrome. This daily injectable pen would come out of patients’ medical savings.
- Ozempic® (Semaglutide), belonging to Novo Nordisk, is a weekly injection, FDA approved for Type 2 Diabetes. Out of all the GLP-1 agonists, the Ozempic® pen has by far seen the most counterfeits, to the extent that SAHPRA issued a media release in December 2023 warning the public and urging them to use registered medicines via licensed pharmacies.Semaglutide is a single receptor agonist targeting GLP-1 receptors.
- Mounjaro® (Tirzepatide), held by Eli-Lilly, initially registered for Type 2 Diabetes in December 2024, was granted approval for medical weight loss by SAHPRA in October 2025. Available in vials and pens with numerous once-weekly dosages available, it makes it the most likely injectable which will be used for microdosing, which is off-label. Mounjaro® is a dual receptor agonist targeting both GLP-1 and GIP (glucose-dependent insulinotropic polypeptide) receptors. Because of its dual action, it can achieve greater weight-loss results ranging from 15-26% body weight.
- Wegovy® (Semaglutide) launched in SA in August 2025 by Novo Nordisk with the indication for medical weight loss. Considered to be the “big Ozempic® pen”, it allows for increased doses specifically for the weight-loss indication.
Semaglutide, Tirzepatide and Retatrutide (to be released in 2026) side effects remain similar. They are predominantly gastrointestinal side effects of nausea, constipation and diarrhoea. Titrating the dosage very slowly usually mitigates these symptoms. Slow and steady often wins the race. Rashes have been reported. Less commonly reported is hair loss.
Registered GLP-1 Agonists in South Africa (as of 2025):
| Brand Name | Generic Name | Company | Indication (SAHPRA) | Dosing Frequency |
| Byetta® | Exenatide | AstraZeneca | Type 2 Diabetes | Twice daily injection |
| Bydureon BCise® | Exenatide ER | AstraZeneca | Type 2 Diabetes | Once weekly injection |
| Victoza® | Liraglutide | Novo Nordisk | Type 2 Diabetes | Once daily injection |
| Trulicity® | Dulaglutide | Eli Lilly / Aspen | Type 2 Diabetes | Once weekly injection |
| Saxenda® | Liraglutide 3 mg | Novo Nordisk | Chronic weight management | Once daily injection |
| Ozempic® | Semaglutide | Novo Nordisk | Type 2 Diabetes | Once weekly injection |
| Wegovy® | Semaglutide | Novo Nordisk | Chronic weight management | Once weekly injection |
| Mounjaro® | Tirzepatide | Eli Lilly / Aspen | Type 2 Diabetes & Weight management | Once weekly injection |
Let’s have a look at the criteria for Obesity and Overweight
Although we know that BMI is an imperfect tool, it remains useful. A BMI ≥ 30kg/m² (obese) or ≥ 27kg/m² (overweight). The measurement is calculated with weight in kilograms divided by height in metres squared.
A Waist-to-Hip Ratio (WHR) of ≥ 0.85 for females and ≥ 0.9 for males is an indication of higher health risks. Take your waist circumference at the smallest part of your waist and hip circumference at the widest part of your buttocks. Use a tape measure and do not pull too tightly. Use the same unit of measure: centimetres or inches. Divide the waist circumference by the hip circumference and that is your WHR.
Body composition data in conjunction with these other measurements, are useful as diagnostic and monitoring tools. A comprehensive report of your BMI, body fat % and muscle mass are included in the report. The In Body Scan® is such a device.


What are potential concerns
Counterfeit products with potentially harmful substances or insulin are flagrantly being sold on the black market, placing the public at great risk.
Will there be loss of muscle mass? We must stress the importance of adequate protein intake and adequate resistance training to maintain muscle mass and then monitoring is crucial.
Specialised doctor-managed weight-loss centres with equipment to assess body composition become vital. We are aiming not only for a normalised BMI or WHR but also for a decline in body fat percentage and a reduction in visceral fat. Tracking the patient’s muscle mass becomes vital because we must avoid a condition called sarcopenia. The body naturally loses 3-5% muscle mass per decade, but it accelerates after the age of 60.
I personally wonder if GLP-1s will affect the absorption of other medications like hormone supplementation, for example, micronised progesterone taken at night for sleep in perimenopause and menopause. Individual patient experiences may vary.
Patients must be adequately managed and followed up to discuss sustainability, discontinuation and the potential for rebound weight gain.
Lifelong treatment remains crucial for patients diagnosed as obese or overweight. A paradigm shift is needed around the chronicity of both these diseases. We would not stop medication prescribed for hypertension and, in the same vein, why stop GLP-1 agonists when they are life-saving for the obese and overweight. Will medical aids get on board with funding these treatments in time? Will it be sustainable for people if paid out of pocket?
WHAT DOES THE FUTURE HOLD?
In the pipeline
- Semaglutide (Rybelsus), FDA approved in 2019, registered in SA in 2024 but not yet sold locally, promises a once-a-day dosing in tablet form, making it much easier for patients travelling without the need for refrigeration. It will control blood glucose levels and reduce the risk of cardiovascular disease.
- Retatrutide is still in its research phase. Developed by Eli-Lilly, it is recognised to be a triple receptor agonist, containing glucagon-like peptide-1, GIP (glucose-dependent insulinotropic polypeptide) and glucagon (GCGR). In studies, it has shown impressive efficacy in weight loss. The mean weight loss has exceeded 17.5% with up to 24.2% weight loss reported over 48 weeks at the higher doses. It is currently in Phase 3 clinical trials. By targeting 3 hormone pathways, it promises profound weight-loss results and overall health benefits, more than the single or dual agonists achieve.
- Other peptides: MOTS-c, in its research phase, promises to be a game-changer for increasing muscle mass, especially in conjunction with GLP-1 agonists. Developed specifically for patients with sarcopaenia, this peptide holds great promise for restoring mitochondrial function, among many other benefits. Further clinical trials are needed to establish treatment protocols and confirm efficacy in humans.
The next generation of incretin-based therapies promises deeper metabolic reprogramming, targeting not just glucose and weight, but cellular energy, muscle preservation, and potentially ageing itself.
Microdosing will become more topical for patients classified as “skinny fat”: being perceived to be healthy, but with low muscle mass and high visceral fat. Dosing regimens for longevity vs diabetes will also become topical and will need to be standardised for longevity benefits.
The use in perimenopause and menopause, where there is a redistribution of fat, weight gain and inflammation.
We need to maintain a critical view of on- and off-label indications and athletic use of all peptides. The World Anti-Doping Agency’s (WADA) stance on peptide and sporting use. Unregulated use in gyms remains a concern and how we navigate this in SA will be interesting.
It remains that there must be emphasis on lifestyle foundations rather than pharmaceuticals. We need a cautious approach to growth hormone and other peptide therapies which can be used as surgical recovery tools rather than anti-ageing panaceas. We need precision medicine rather than broad, indiscriminate use.
Their role in addictive disorders and restrictive eating disorders, if any, and Body Dysmorphia in the future will become clearer.
Within the realm of longevity medicine, health span, not just lifespan, is a shift in paradigm.
General advice
The responsible use of GLP-1s is very important.
As Schedule 4 medications, they are doctor-prescribed, requiring a comprehensive consultation, assessment and blood-panel work-up with nutritional advice, nutrient replenishment, muscle preservation and hormone balancing.
There are contraindications to the use of GLP-1s. Consult with your doctor to determine if they are safe for you.
We must banish misuse and misunderstanding about this class of medications and use them responsibly because they are here to stay.
Avoid at all costs
- Counterfeit products available online and on the black market (they can contain dangerous, unknown substances or insulin).
- Hairdressers, personal trainers or unqualified individuals should not be selling GLP-1s.
- Friendly pharmacists dispensing GLP-1s without a doctor’s prescription.
In summary
For decades, those who have struggled with obesity and being overweight have lived with a burden of stigma.
GLP-1s offer people hope. Appetite regulation vs willpower will reframe the guilt and shame around eating. This heralds a new era for the doctor-patient partnership.
They are a turning point in how we approach weight and wellness. When combined with a functional medicine approach, they become a tool for sustainable transformation.
About the author
MBChB, Advanced Diploma in Aesthetic Medicine (Cum Laude)
Dr Heidi Frere is the Founder and Medical Director of Best You Medical Wellness & Aesthetics in Douglasdale, Johannesburg. With over 25 years of medical experience, she combines her extensive clinical background with a passion for holistic wellness. Dr Frere holds an MBChB and an Advanced Diploma in Aesthetic Medicine (Cum Laude), and has developed a special interest in medical aesthetics, functional medicine, hormone replenishment, and medical weight management.

